Our Research
Our group is interested in what makes some early prostate cancers become lethal while others remain indolent, and how we can use that knowledge to diagnose, monitor and treat disease more intelligently. We work across tissue, blood and patient-derived models, with a particular focus on epigenetics, cell-free DNA and DNA repair–targeting therapies. We organise our work around four connected themes.
Epigenetic landscapes of early prostate cancer
Genetic mutations only tell part of the story in prostate cancer. We are particularly interested in epigenetic changes – especially DNA methylation – that shape cell identity and behaviour long before a tumour becomes clinically obvious. Using bulk and single-cell methylation profiling in multi-focal and early lethal prostate cancers, we:
map truncal methylation “barcodes” that are shared across different tumour foci and appear early in the life of a cancer;
define epigenetic programmes linked to neuronal plasticity and tumour–microenvironment cross-talk, which associate with worse clinical outcomes;
build cell-type–resolved methylation atlases to understand how luminal cells, immune cells, fibroblasts and other compartments change as disease progresses.
Key questions we are asking
Which epigenetic changes define an “early lethal” trajectory, and can they be detected years before metastasis?
How do neuronal and microenvironmental programmes emerge in apparently localised disease?
Can epigenetic signatures help us distinguish cancers that need treatment from those that can be safely watched?
Liquid biopsies and cell-free DNA biomarkers
Repeated biopsies of the prostate are invasive and imperfect. We are therefore developing liquid biopsy approaches, focusing on methylation-based profiling of cell-free DNA (cfDNA) in blood. Building on our existing platforms and related work, we:
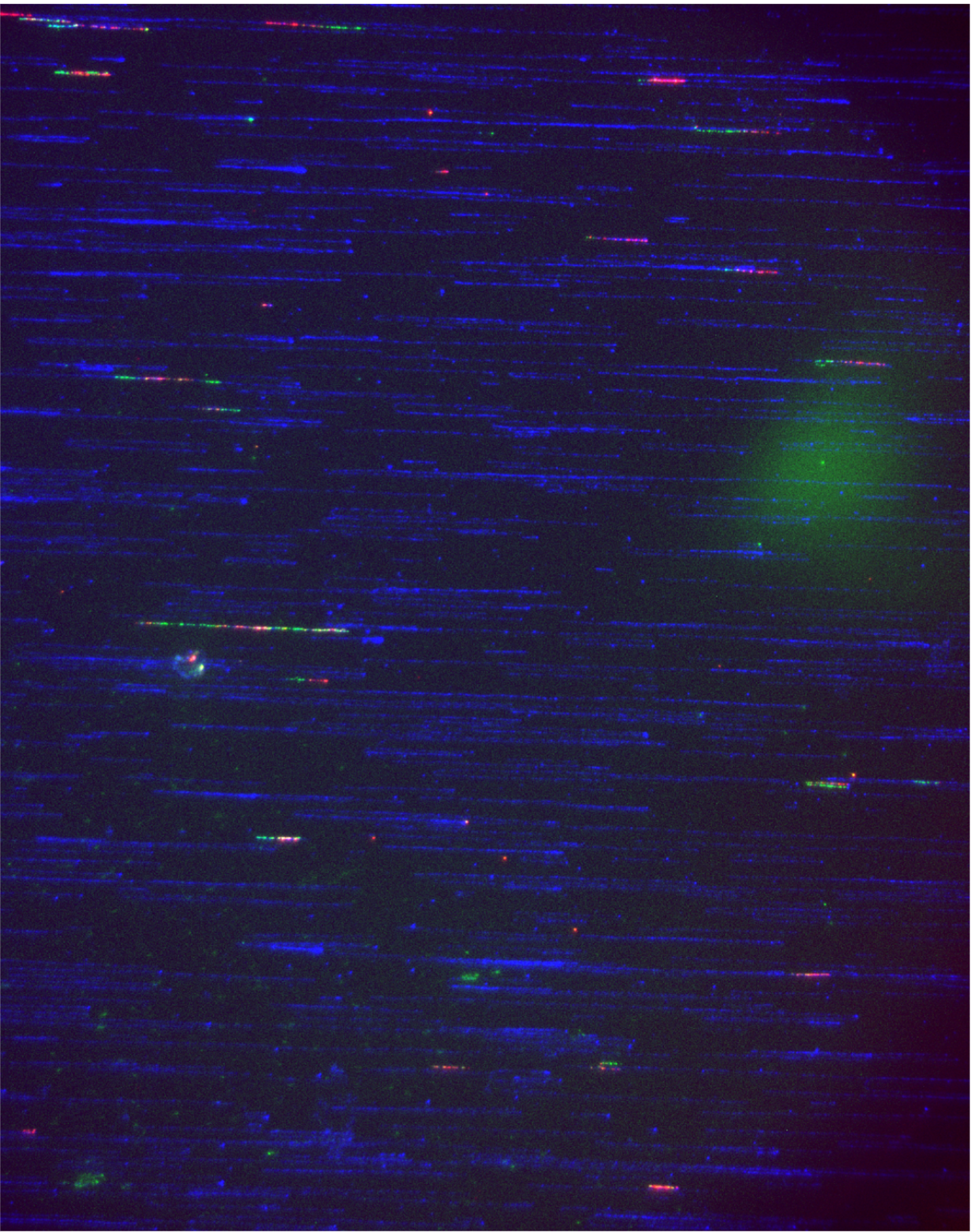
design targeted methylation panels that capture tumour-specific differentially methylated regions (DMRs) at single-base resolution;
use concepts such as epiallele (epiallele) frequency to sensitively detect rare tumour-derived molecules in plasma;
deconvolve cfDNA to separate luminal tumour DNA from circulating microenvironmental DNA (cmDNA) shed by tumour-educated immune and stromal cells.
Our goal is to translate these tools into blood tests that can aid:
early detection of clinically significant prostate cancer,
risk stratification after surgery or radiotherapy, and
real-time monitoring of treatment response and resistance in trials and routine care.
Key questions we are asking
How early can methylation-based liquid biopsies detect aggressive prostate cancer?
Can cfDNA patterns tell us who is likely to recur after apparently curative treatment?
What does the presence of tumour-educated immune cell DNA (cmDNA) in plasma reveal about response to therapy?
DNA repair, therapeutic mechanisms and earlier treatment
Drugs that target DNA damage response (DDR) pathways – particularly PARP inhibitors – were developed for advanced cancers with defects in genes such as BRCA1/2. We are interested in how these agents work at a mechanistic level in prostate cancer, and how to use them more intelligently in both BRCA-mutant and BRCA-proficient settings.
Our work spans:
dissecting how PARP and androgen receptor (AR) signalling intersect;
using functional genomics to identify factors that modulate sensitivity or resistance to treatments of interest;
understanding why some DNA repair gene–proficient tumours still respond to PARP combinations, and how to identify those patients in advance.
A central principle is to bring powerful treatments earlier in the disease course for the right men. We use surgical window-of-opportunity trials around prostatectomy, coupled with intensive tissue and plasma sampling, to see how these drugs perturb tumour and microenvironmental biology in real time.
Key questions we are asking
Which biological features define men most likely to benefit from PARP-based strategies in early disease?
How do DDR-targeting drugs reshape cell fate in prostate tumours?
Can we propose biomarkers, and use short pre-surgical “windows” to test and refine rational drug combinations before larger trials?
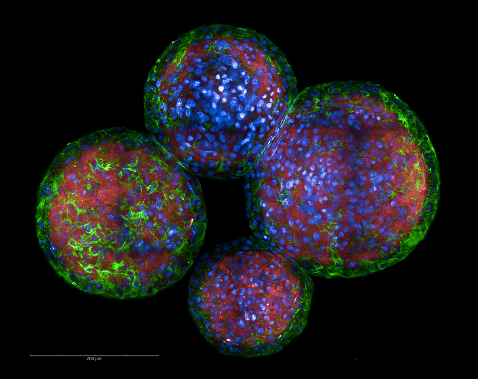
Patient-derived models and trial-linked platforms
Drugs that target DNA damage response (DDR) pathways – particularly PARP inhibitors – were developed for advanced cancers with defects in genes such as BRCA1/2. We are interested in how these agents work at a mechanistic level in prostate cancer, and how to use them more intelligently in both BRCA-mutant and BRCA-proficient settings.
Our work spans:
dissecting how PARP and androgen receptor (AR) signalling intersect;
using functional genomics to identify factors that modulate sensitivity or resistance to treatments of interest;
understanding why some DNA repair gene–proficient tumours still respond to PARP combinations, and how to identify those patients in advance.
A central principle is to bring powerful treatments earlier in the disease course for the right men. We use surgical window-of-opportunity trials around prostatectomy, in partnership with Dr Simon Pacey (Consultant Medical Oncologist and lead for the early-phase prostate cancer trials portfolio), coupled with intensive tissue and plasma sampling within our PICASSO bioethical framework, to see how these drugs perturb tumour and microenvironmental biology in real time.
Key questions we are asking
Which biological features define men most likely to benefit from PARP-based strategies in early disease?
How do DDR-targeting drugs reshape cell fate in prostate tumours?
Can we propose biomarkers, and use short pre-surgical “windows” to test and refine rational drug combinations before larger trials?